Common Cell Staining Techniques
Coloring the animal cell – Effective visualization of animal cell components is crucial for cytological analysis and diagnosis. Achieving this requires employing various staining techniques, each with its own strengths and weaknesses depending on the specific cellular structures of interest and the desired level of detail. The choice of staining method is dictated by the research question and the nature of the cellular components being investigated.
Comparison of Cell Staining Techniques
The selection of an appropriate staining technique depends heavily on the specific cellular structures one wishes to highlight. Several common methods exist, each utilizing different chemical principles to achieve selective staining. The following table compares four widely used techniques: Hematoxylin and Eosin (H&E), Wright’s stain, Giemsa stain, and Periodic Acid-Schiff (PAS) stain.
| Staining Technique | Target Structures | Staining Mechanism | Advantages/Disadvantages |
|---|---|---|---|
| Hematoxylin and Eosin (H&E) | Nuclei (hematoxylin), cytoplasm and extracellular matrix (eosin) | Hematoxylin, a basic dye, binds to negatively charged DNA in the nucleus; eosin, an acidic dye, binds to positively charged proteins in the cytoplasm. | Widely used, inexpensive, provides good overall tissue morphology; can mask some cellular details. |
| Wright’s Stain | Blood cells (leukocytes, erythrocytes) | A mixture of eosin and methylene blue, differentiating blood cell types based on their cytoplasmic and nuclear components. | Excellent for differentiating blood cell types; less useful for other tissues. |
| Giemsa Stain | Chromosomes, parasites, blood cells | Similar to Wright’s stain, uses a mixture of methylene blue and eosin; stains DNA and RNA differentially. | Excellent for visualizing parasites and differentiating blood cell types; can be time-consuming. |
| Periodic Acid-Schiff (PAS) | Carbohydrates, glycoproteins, glycogen | Periodic acid oxidizes carbohydrates, forming aldehydes that react with Schiff’s reagent, producing a magenta color. | Specifically stains carbohydrates; useful for identifying glycogen storage diseases and certain types of cancer; requires careful control of reaction conditions. |
Chemical Principles of Hematoxylin and Eosin Staining
Hematoxylin and eosin staining relies on the electrostatic interaction between charged dyes and cellular components. Hematoxylin, a basic dye, carries a positive charge and binds to the negatively charged phosphate groups of DNA within the cell nucleus, resulting in a dark purple or blue stain. Eosin, an acidic dye, carries a negative charge and binds to positively charged proteins in the cytoplasm and extracellular matrix, staining these structures pink or red.
This differential staining allows for easy visualization of the nucleus and the surrounding cytoplasm.
Chemical Principles of Giemsa Staining
Giemsa stain, a polychromatic stain, contains a mixture of methylene blue, azure B, and eosin. Methylene blue and azure B are cationic dyes that bind to negatively charged components like nucleic acids (DNA and RNA) and acidic proteins. The interaction is based on electrostatic attraction. Eosin, an anionic dye, stains basic components like cytoplasm. The different components of Giemsa stain interact with various cellular structures at different affinities, producing a differential staining pattern that highlights various cellular features, particularly useful in identifying blood cell types and parasites.
Hematoxylin and Eosin Staining Procedure
Preparing an animal cell slide for H&E staining involves several steps:
1. Tissue Fixation
The tissue sample is fixed using a fixative like formalin to preserve its structure and prevent degradation.
2. Tissue Processing
The fixed tissue is processed through a series of graded alcohols and xylenes to dehydrate it and make it suitable for paraffin embedding.
3. Paraffin Embedding
The dehydrated tissue is embedded in paraffin wax to provide support during sectioning.
4. Sectioning
Thin sections (typically 5-7 μm) are cut using a microtome.
5. Slide Mounting
The sections are mounted onto glass slides.
6. Deparaffinization and Rehydration
The paraffin wax is removed, and the sections are rehydrated through a series of graded alcohols.
7. Hematoxylin Staining
The sections are stained with hematoxylin to stain the nuclei.
8. Differentiation
Excess hematoxylin is removed using a differentiating agent like acid alcohol to prevent overstaining.
9. Eosin Staining
The sections are stained with eosin to stain the cytoplasm and extracellular matrix.1
0. Dehydration and Mounting
The stained sections are dehydrated, cleared in xylene, and mounted with a mounting medium.
Specific Organelle Staining
Selective staining techniques are crucial for visualizing specific organelles within the complex milieu of the animal cell. These methods exploit the biochemical properties of organelles, allowing researchers to isolate and highlight them for detailed study of structure and function. The choice of stain depends heavily on the target organelle and the desired level of detail.
Nucleus Staining
The nucleus, the cell’s control center, is readily stained using dyes that bind to DNA. DAPI (4′,6-diamidino-2-phenylindole) is a widely used fluorescent stain that intercalates between DNA base pairs, emitting a bright blue fluorescence upon excitation with ultraviolet light. Other common nuclear stains include hematoxylin, which binds to negatively charged DNA and stains the nucleus a dark purple or blue, and Hoechst stains, which also bind to DNA and exhibit fluorescence in the ultraviolet range.
The intensity of the stain can often reflect the level of DNA condensation, providing insights into the cell cycle stage. For instance, a highly condensed nucleus, as seen during mitosis, would stain more intensely than a less condensed nucleus in interphase.
Mitochondria Staining
Mitochondria, the powerhouses of the cell, can be visualized using several techniques. MitoTracker dyes are a popular choice; these are lipophilic cationic dyes that accumulate in mitochondria due to the high membrane potential across the mitochondrial inner membrane. Different MitoTracker dyes emit fluorescence at various wavelengths, allowing for multiplexing with other stains. Another approach involves using antibodies against mitochondrial proteins, coupled with fluorescent secondary antibodies, enabling highly specific mitochondrial labeling.
The intensity and distribution of the mitochondrial stain can reflect the metabolic activity of the cell; cells with high metabolic demands will often exhibit a more intense and diffuse staining pattern compared to cells with lower metabolic activity.
Endoplasmic Reticulum Staining
The endoplasmic reticulum (ER), a vast network of membranes involved in protein synthesis and lipid metabolism, can be stained using various methods. One common approach utilizes fluorescent dyes that bind to specific ER proteins, such as the ER chaperone protein BiP (Binding immunoglobulin protein). Alternatively, dyes that specifically label the ER membrane can be used. These stains can reveal differences in ER morphology, such as the presence of extensive rough ER (studded with ribosomes) in protein-secreting cells versus a more prominent smooth ER in cells involved in lipid metabolism.
So, you’re coloring an animal cell, huh? Pretty basic stuff, right? Mitochondria, ribosomes – the usual suspects. But let’s be honest, sometimes you need a break from the microscopic world. Check out these awesome circus animal train coloring pages for a fun change of pace before diving back into the nucleus and cytoplasm.
It’s a good way to unleash your inner child before tackling those pesky Golgi apparatuses again.
The overall staining pattern can provide valuable information on the cell’s synthetic capacity and metabolic state.
Golgi Apparatus Visualization: A Hypothetical Experiment
To visualize the Golgi apparatus, we could design an experiment using a fluorescently labeled lectin. Lectins are carbohydrate-binding proteins that can specifically bind to the glycosylated proteins found in the Golgi apparatus. For example, we could use Alexa Fluor 488-conjugated wheat germ agglutinin (WGA), a lectin that binds to N-acetylglucosamine and sialic acid residues. Cells would be incubated with the labeled WGA, followed by fixation and imaging using fluorescence microscopy.
The expected results would show a distinct perinuclear localization of bright green fluorescence, corresponding to the characteristic location of the Golgi apparatus. The intensity of the fluorescence could potentially indicate the level of protein glycosylation activity within the Golgi. Variations in Golgi morphology, such as fragmentation or expansion, could also be observed, potentially reflecting changes in cellular stress or function.
Organelle Staining Variations Across Cell Types
Different animal cell types exhibit variations in organelle structure and function that can be revealed through staining techniques. For example, neurons, with their extensive axonal transport systems, will show a markedly different distribution of mitochondria compared to epithelial cells. Similarly, the abundance and morphology of the ER will vary significantly between cells specialized in protein secretion (e.g., plasma cells) versus cells with a primary role in lipid metabolism (e.g., hepatocytes).
These differences in organelle structure and distribution are reflected in the staining patterns, providing valuable insights into the cellular specialization and function. For instance, comparing the mitochondrial staining intensity in a cardiomyocyte (heart muscle cell) versus a fibroblast (connective tissue cell) would reveal the significantly higher metabolic activity of the cardiomyocyte, reflected in a more intense and widespread mitochondrial stain.
Microscopy and Image Analysis: Coloring The Animal Cell
Visualizing the intricate details of stained animal cells requires sophisticated microscopy techniques coupled with robust image analysis methods. The choice of microscopy depends heavily on the specific structures of interest and the desired level of detail. Different microscopy approaches offer varying resolutions and capabilities, influencing the effectiveness of visualizing stained cellular components.
Different types of microscopy offer unique advantages in visualizing stained animal cells. Light microscopy, the most basic form, uses visible light to illuminate the sample, providing a relatively low-resolution image suitable for observing the overall cell structure and larger organelles. Fluorescence microscopy, however, utilizes fluorescent dyes to label specific cellular components, allowing for the visualization of particular structures with higher specificity.
This technique is particularly useful for studying the localization of proteins or other molecules within the cell. Electron microscopy, on the other hand, employs a beam of electrons to create an image, achieving significantly higher resolution than light microscopy. This allows for the visualization of extremely fine details, such as the internal structure of organelles, but requires extensive sample preparation and can be more expensive.
Microscopy Techniques and Their Applications
The following table summarizes the resolution capabilities and applications of different microscopy techniques in visualizing stained animal cells. The resolution values are approximate and can vary depending on the specific instrument and settings.
| Microscopy Type | Resolution (nm) | Applications in Visualizing Stained Animal Cells | Advantages/Disadvantages |
|---|---|---|---|
| Light Microscopy | 200-500 | Observing overall cell morphology, visualizing stained nuclei and larger organelles (e.g., mitochondria, lysosomes with appropriate stains). | Relatively inexpensive and easy to use; lower resolution limits detail. |
| Fluorescence Microscopy | 200-300 | Localizing specific proteins or other molecules using fluorescently labeled antibodies or dyes; visualizing the distribution of stained organelles. | High specificity; allows for multiplexing (staining with multiple fluorophores); can be sensitive to photobleaching. |
| Transmission Electron Microscopy (TEM) | 0.1-0.2 | Visualizing the ultrastructure of organelles (e.g., ribosomes, membranes); detailed analysis of stained cellular components at the nanometer scale. | Extremely high resolution; requires extensive sample preparation (often involving heavy metal staining); expensive. |
| Scanning Electron Microscopy (SEM) | 1-10 | Imaging the surface of cells and observing three-dimensional structures; less useful for visualizing internal stained structures. | Provides high-resolution surface images; less effective for internal cellular structures; sample preparation may involve coating with conductive material. |
Image Processing Techniques
Raw microscopic images often require processing to enhance the visualization of stained structures. Several image processing techniques are commonly employed to improve image quality and extract meaningful information. These techniques can significantly improve the clarity and interpretability of the data obtained.
Common techniques include adjustments to brightness and contrast, background subtraction to reduce noise, deconvolution to sharpen the image and improve resolution, and segmentation to identify and separate different stained structures within the cell. Advanced techniques like 3D reconstruction from multiple image stacks can provide a comprehensive view of the three-dimensional organization of stained components within the cell. For instance, deconvolution algorithms can computationally remove out-of-focus blur, thereby improving the resolution and revealing finer details of stained structures that might be obscured in the original image.
Similarly, segmentation techniques, often involving thresholding and edge detection, can help isolate specific stained organelles from the background, allowing for quantitative analysis of their size, shape, and distribution.
Interpreting Stained Cell Images
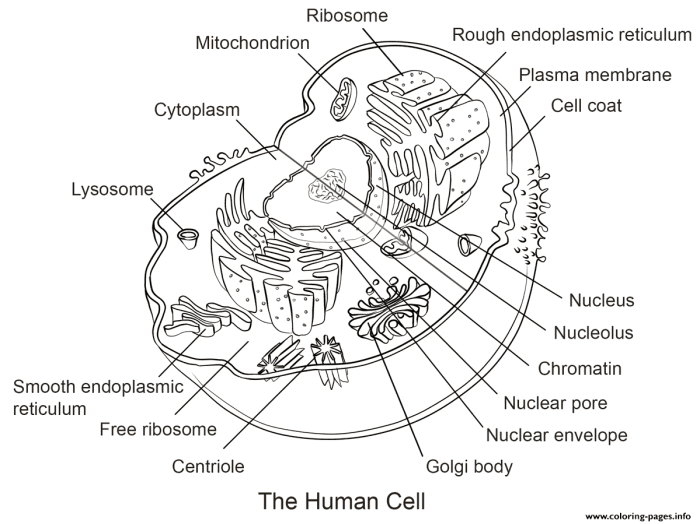
Accurate interpretation of stained animal cell images is crucial for cellular biology research. The success of this interpretation hinges on understanding both the staining process and potential sources of error, allowing for the differentiation of genuine cellular structures from artifacts. Careful observation, combined with knowledge of cell biology, is essential for drawing valid conclusions from microscopic images.
Common Staining Artifacts and Mitigation Strategies
Several factors can introduce artifacts into stained cell images, leading to misinterpretations. These artifacts can arise from various stages of the staining and microscopy process. For instance, poor fixation can cause cell shrinkage or distortion, altering the true morphology of organelles. Crystallization of the stain itself can appear as spurious structures within the cell. Furthermore, uneven staining can lead to inaccurate assessments of organelle size and distribution.
To minimize these artifacts, meticulous attention to detail is paramount. Proper fixation protocols, using appropriate fixatives and durations, are essential. Careful control of staining parameters, including concentration, time, and temperature, can also significantly reduce the incidence of artifacts. Finally, employing standardized staining and imaging procedures allows for better reproducibility and reduces the likelihood of introducing biases.
The use of positive and negative controls during the staining process can also aid in identifying and distinguishing true signals from artifacts.
Interpreting a Stained Animal Cell Image: Organelle Identification
A typical stained animal cell image, viewed under a light microscope, reveals a variety of structures. For example, the nucleus, typically stained a dark purple or blue with hematoxylin, appears as a large, round or oval structure, often centrally located. Its prominent nucleolus, a smaller, intensely stained region within the nucleus, is involved in ribosome synthesis and may appear as a dark spot within the larger nuclear structure.
The cytoplasm, often stained a lighter pink or red with eosin, encompasses the rest of the cell and contains various organelles. The endoplasmic reticulum, depending on the staining technique, might be visible as a network of interconnected tubules and sacs. Its appearance can vary; a smooth ER might be less prominent than a rough ER, which is often studded with ribosomes, giving it a granular appearance.
Mitochondria, stained with specific mitochondrial stains (such as Janus Green B), appear as elongated or oval structures, often scattered throughout the cytoplasm. Their size and shape can vary, reflecting their dynamic nature within the cell.
Detailed Description of a Typical Stained Animal Cell Image
At low magnification (e.g., 4x or 10x), the overall cell shape and size become apparent. The nucleus is easily identifiable as a large, dark-stained structure. At higher magnification (e.g., 40x), the nuclear membrane, nucleolus, and chromatin (DNA) may become visible within the nucleus. The cytoplasm appears as a lighter-stained background, with some organelles potentially visible as small, distinct structures.
At the highest magnifications (e.g., 100x with oil immersion), the fine details of organelles like mitochondria and the endoplasmic reticulum might become visible, though their identification may require specific staining techniques to enhance their contrast and visibility. The color of the organelles depends on the stain used; for example, using hematoxylin and eosin staining, the nucleus would appear dark purple/blue, while the cytoplasm would appear pink/red.
Mitochondria, if stained specifically, would exhibit their characteristic color according to the chosen stain. The overall morphology of the cell, including the shape and relative size of organelles, should be consistent with the cell type being examined. Deviations from the expected morphology may indicate artifacts or cellular abnormalities. For instance, a shrunken or distorted cell might indicate issues with the fixation process.
Uneven staining may suggest problems with the staining technique itself.
Applications of Animal Cell Staining
Animal cell staining is a cornerstone technique across diverse biological fields, providing invaluable insights into cellular structure, function, and pathology. Its applications span from routine medical diagnostics to cutting-edge research investigations, enabling a deeper understanding of life at the cellular level. The choice of staining method is crucial, dictated by the specific research question or diagnostic goal.
The power of staining lies in its ability to highlight specific cellular components, revealing details otherwise invisible under standard microscopy. This selective enhancement allows researchers and clinicians to identify abnormalities, track dynamic processes, and ultimately, improve diagnostic accuracy and therapeutic strategies.
Medical Diagnosis Using Animal Cell Staining
Animal cell staining plays a crucial role in various medical diagnostic procedures. Blood smears, for example, are routinely stained using techniques like Giemsa or Wright staining to differentiate various blood cell types, aiding in the diagnosis of conditions such as anemia, leukemia, and infections. The distinct staining patterns of different blood cells—the characteristic pink and purple hues of erythrocytes and leukocytes, respectively—allow for rapid and accurate identification of abnormalities in their numbers or morphology.
Similarly, tissue biopsies are stained using hematoxylin and eosin (H&E) staining, a standard histological technique that differentiates between cell nuclei (stained blue/purple by hematoxylin) and cytoplasm (stained pink/red by eosin). This staining method is essential for cancer diagnosis, enabling pathologists to identify malignant cells based on their size, shape, and arrangement within the tissue. The identification of abnormal cell morphology in biopsies, such as increased nuclear-to-cytoplasmic ratio or abnormal chromatin distribution, are key indicators of cancerous growth.
Animal Cell Staining in Biological Research
Beyond medical diagnostics, animal cell staining is an indispensable tool in various biological research areas. In studies of cell division, fluorescent dyes such as DAPI (4′,6-diamidino-2-phenylindole) are used to visualize DNA, allowing researchers to track chromosome segregation and identify potential errors in mitosis or meiosis. These observations are vital for understanding the mechanisms of cell division and identifying potential causes of genetic disorders.
Similarly, studies of cell signaling often employ immunofluorescence techniques. These techniques utilize antibodies conjugated to fluorescent dyes to specifically target and visualize proteins involved in signaling pathways. By observing the localization and changes in the intensity of fluorescent signals, researchers can gain insights into the dynamics of cellular communication and response to various stimuli. This method is essential for investigating the roles of specific proteins in cell growth, differentiation, and response to disease.
Furthermore, animal cell staining techniques are crucial in understanding disease mechanisms. For example, staining techniques can be used to identify and quantify the presence of specific pathogens within infected cells or to visualize the accumulation of misfolded proteins associated with neurodegenerative diseases.
Visualization of Cellular Processes Using Fluorescent Dyes, Coloring the animal cell
Fluorescent dyes offer a powerful means of visualizing specific cellular processes in living animal cells. These dyes are designed to bind to particular molecules or structures within the cell, emitting light at specific wavelengths when excited by a light source. Live-cell imaging using fluorescent dyes allows researchers to observe dynamic processes in real-time, providing insights into the temporal and spatial aspects of cellular events.
For instance, the use of fluorescent calcium indicators allows researchers to monitor changes in intracellular calcium concentrations, a key signaling molecule involved in numerous cellular processes, including muscle contraction, neurotransmission, and apoptosis. Similarly, fluorescent probes can be used to track the movement of organelles within the cell, providing insights into intracellular trafficking and transport mechanisms. The non-invasive nature of many fluorescent dyes allows for long-term observation of cellular processes without significantly affecting cell viability, providing a valuable tool for studying cellular dynamics under various conditions.
